ð
In
order to get satisfactory results in dyeing, textile auxiliaries are invariably
employed.
ð
The
use of particular auxiliary in dyeing will depend upon the type of dyestuff and
also on the type of fibre.
ð The important
auxiliaries used in dyeing can be broadly grouped into the following classes:-
(a)
Wetting
and penetrating agents.
(b)
Dispersing
agents.
(c)
Levelling
agents.
(d)
Sequestering
agents.
(e)
Antifoaming
agents.
(f)
Accelerators.
(g)
Migration
Inhibitors.
(h)
Dye
fixing agents.
(i)
After-washing
agents.
(j)
Stripping
agents.
Wetting
and Penetrating Agents
ð Wetting agents are
added to the dye bath to ensure that the entering goods are thoroughly and uniformly
wetted with the dye solution.
ð In this capacity they
are often referred to as Penetrating agents, since they cause the dye solution
to penetrate into the interior of the yarn.
ð It should be noted that
they have little effect on the rate at which dye molecules migrate into the fibre substance.
ð
They also reduce the interfacial tension between oil and water by
adsorbing at the liquid-liquid interface. Many surfactants can also assemble in
the bulk solution into aggregates.
ð
Examples of such aggregates are vesicles and micelles. The concentration at which wetting agent
begin to form micelle is known as the critical micelle concentration (CMC).
ð
When micelles form in water, their tails form a core that can
encapsulate an oil droplet, and their (ionic/polar) heads form an outer shell
that maintains favourable contact with water.
ð
When wetting agents assemble in oil, the aggregate is referred to as a
reverse micelle.
ð In a reverse micelle, the heads are in the
core and the tails maintain favourable contact with oil.
ð In the preparation of
solutions of naphthols and vat dyes wetting agents are used for pasting the
dyestuff.
ð Sulphated oils have
proved to be satisfactory wetting agents in the application of vat and azoic
dyes by padding techniques.
ð The wetting agents help
to prevent dusting off of the materials. The wetting power further increased by
adding electrolytes.
ð The dyeing of polyester
and polyester/cellulosic blends with disperse dyes, the use of wetting agents
is desirable, since polyester fibres being hydrophobic are difficult to wet.
ð Anionic as well as
certain non-ionic wetting agents of polyethylene glycol type are effective in
the dyeing methods wherein exhaustion takes place.
ð In the continuous
thermofixation dyeing of polyester and P/C blends, the selection of a proper
wetting agent is of the utmost importance.
ð Anionic highly
sulphonated oils such as ‘Calsolene oil HSI” have been found to be efficient
wetting agents, while non-ionic wetting agents are less satisfactory.
ð Non-ionic gives less
colour yield because of the formation of complexes with the dye, which have a
lower rate of vapourisation as compared to the dye alone.
ð Sulphated Vegetables
Oils:- Sulphated castor oil (Turkey Red Oil) is a good wetting agent. Its main
constituent is sulphated rincinoleate.
Chemical
Formula of Turkey Red Oil
ð Sulphated Fatty Acid
Esters:- Sulphated methyl oleate (or butyl oleate) is also a good anionic
wetting agent.
ð The most powerful
wetting agent is Dioctyl sulphosuccinate (Octyl being obtained by 2 – ethyl
hexanol).
Me
= Methyl (CH3)
Chemical
Formula of Dioctyl Sulphosuccinate
ð Fatty acid amides and
their derivatives, Alkyl phenol condensates are also good wetting agents.
Dispersing
Agents
ð
The
function of a dispersing agent is to prevent agglomeration of individual dye
particles during dyeing.
ð
Dispersing
agents enhance the dispersion and ensure a fine particle size. It enhances the
processes, by effectively dispersing finely divided solids and liquids in
aqueous environment.
ð
The
nature of dispersing agent depends upon the properties desired and class of the
dyes. It can be of both high and low molecular weights.
ð
Today,
not only do these dispersing agents have a strong dispersing effect, but also
exhibit very high temperature stability throughout the dyeing cycle.
ð
Dispersing
agents are of particular interest in the application of vat dyes by vat acid
and pigment padding methods.
ð
This
product is variously described as sodium salt of a naphthalene sulphonic acid
condensation product (Setamol WS of BASF India Ltd.), aromatic sulphonic acid
condensation product (Uniperse P), disodium salt of methylene dinaphthalene disulphonic
acid (Dadamol V).
Chemical Formula of Dadamol V
ð
These
are available in the form of yellowish brown powder, light beige coloured
powder or dark brown mobile liquid.
ð
The
powder brand is a non-hygroscopic powder that is readily soluble in water with
any degree of hardness.
ð
It is
resistant to alkalis, acids and salts and has dispersing and protective colloid
properties. It is not a surface active agent and hence has no wetting, foaming
and detergent properties.
ð
Being
anionic in nature, it is compatible with anionic and non-ionic products. When
mixed with cationic products, it may form precipitates.
ð
A 10
% solution of the powder has a pH of 6.5 to 7.5 and has good storage stability.
ð
It
finds use in the dyeing of vat, disperse, solubilised vat and azoic colours.
ð
Disperse
dyes are characterised by low solubility in water and in order to ensure
application of these dyes from aqueous liquors dispersing agents are added to
the dyes as they are marketed.
ð
In
addition to this, it is the usual practice to add some dispersing agent to the
dye bath.
ð
Because
of its non-foaming nature, it is recommended for high temperature dyeing.
ð
Anionic
agents such as sodium dinaphthylmethane sulphonates and lingo sulphonates are
generally used as dispersing agent in polyester dyeing.
ð
When
added to the developing baths in azoic dyeing, the dispersing agent increase
the clarity of these baths and yields dyeing of improved rubbing fastness.
Levelling
Agents
ð The levelness of a
dyeing is generally governed by two properties of the dye.
(a)
The
exhaustion behaviour
(b)
The
levelling out capacity (migration power)
ð One of the important
objectives in dyeing is to secure level or uniform dyeing.
ð Many dyestuffs have a
high initial rate of dyeing and show a tendency to rush on to the fibre,
causing uneven dyeing.
ð Levelling agents are,
therefore, added to the dye bath to regulate the process of dyeing and to get
uniform results.
ð A levelling agent
therefore act on the basis of the following two methods:-
(a)
Allow
the dye to go into the fibre, regardless of the initial unlevelness. During the
course of the further dyeing process, the unlevelness is eliminated by
migration.
(b)
Control
the rate of strike of the dye from the beginning so that it does not go on to
the fibre too rapidly.
ð However, during actual
dyeing a levelling agent generally acts in both ways. These agents are
generally used in dyeing polyester with disperse dye and cellulosics with vat
dyes.
ð In the dyeing of wool
with neutral dyeing acid dyes, it is usually difficult to obtain even dyeing.
ð For a long time anionic
surfactant such as fatty alcohols sulphates, fatty amide sulponates and alkyl
aryl sulphonates were used as levelling agents.
ð These agents compete
with the anionic dyestuffs for the available sites in the fibre and thus
procedure uniform dyeing.
ð Fatty alcohol –
ethylene oxide condensates like cetyl alcohol – ethylene oxide, available in
the liquid form are useful as levelling agents in vat dyeing where the dyeing
rate is very large, as in the case of IN class of vat dyes.
ð Anionic products like
dodecylbenzene sulphonates (Na), sulphated fatty acid esters of lower alcohols,
Turkey Red Oil, fatty alcohol sulphates, etc. may be used as levelling agents
in dyeing acid dyes on nylon.
ð They being anionic in
nature compete with the dye anions for the cationic sites in the fibre and
thereby reduce the effective concentration of the dye anions in the dye bath.
ð However, at the later
stages of dyeing, especially at higher temperatures, the levelling
agents anions are replaced by the dye anions
and good colour value is obtained.
ð Cationic
retarders, like Quaternary ammonium compounds generally described as higher
alkyl trimethyl ammonium chloride are useful as levelling agents in dyeing
cationic dyes acrylic fibres.
ð In
the dyeing of cotton by direct dye levelling agents are added which promote
levelling by breaking the dye aggregates which migrate slowly.
ð Cationic
surfactants and non-ionic ethylene oxide condensates levelling agents are used
for vat dye.
ð The
non-ionic levelling agents are more widely used today. These products from
colloidal aggregates with part of the leuco vat dye present in the dye liquor
and thus slow down the dyeing process.
ð Glue
as well as lignin sulphonate products prepared from cellulose sulphite waste
liquors, a by product in the paper – making industry, are also sometimes used.
ð The
levelling agent has to be chosen with reference to the dye used.
Sequestering Agents
ð The
presence of metals as salts of iron, copper, zinc, manganese, tin, aluminium
etc., in the dye bath is highly undesirable as it adversely affects the tone
and brightness of the colour.
ð A
very small amount of these metals can cause appreciable effect on dyeing. For
example, little as one part of iron or aluminium in 10 million parts of water
cause a detectable shade change in certain metallised dyes.
ð Sequestering
agents are useful in any textile operation in which metallic impurities generally
present in hard water interfere in the processing.
ð The
functions of sequestering agents are
(a) Combine
with metallic impurities like calcium, magnesium and other heavy metal ions in
hard water.
(b) They
form molecules in which the ions are held so securely (sequestered) that they
can no longer react.
(c) The
sequestering agents prevent salts from recontaminating parts.
(d) The
sequestering agents may also tie up the active chemicals in a detergent that
may decrease the cleaning efficiency and life of a wash bath.
ð Common
sequestering agents are orthophosphate, orthosilicate and phosphates. These are
available in powder form.
ð Ideal
sequestering agent is H.E.D.P. (Hydroxyehtylene phosphonic acid), as the
chelation value does not drop at scouring temperature, while the worst
performer is citric acid.
Chemical Formula of EDTA
ð The
most effective sequestering agent in dyeing is ethylene diamine tetraacetic
acid (EDTA).
ð Calgon
which is sodium hexa – metaphosphate is often used as a sequestering agent.
Antifoaming Agents
The use of wetting
agents in textile processing liquors, coupled with the rapid movement either
fabric or liquor causing extensive agitation results in the formation of foam
or froth.
Chemical formula of Trimethyl Cyclohexanol
ð This
creates problems in the application of dyes by dyeing, specifically; high speed
padding mangles are prone to foaming.
ð In
order to overcome this problem, antifoaming agents are added to these liquors.
ð In
early days, benzene, pyridine, and turpentine were used as antifoaming agents.
However, their efficiency was found to be inadequate.
ð Most
of antifoaming agents which are more efficient are based on silicones.
ð Dimethyl
polysiloxane, trimethyl polysiloxane are used as antifoaming agents.
ð Yet
another new type of antifoaming agents is based on polyaminoalkyl substituted
organo polysiloxanes in which there are 5-10 amino substituted hydrocarbon
radicals distributed over each 100 silicon atoms in the chain molecules.
ð Fatty
alcohol and lower alcohols, cyclohexanols etc., are used as antifoaming agents.
ð n
– Octanol, 2 – ethyl – hexanol, n - Octyl alcohol, n – Decanol, lauryl alcohol,
oleyl alcohol, trimethyl cyclohexanol are most efficient antifoaming agents.
Accelerators or
Carriers
ð Carriers
are substances which accelerate the rate of dyeing of disperse dyes on
polyester fibre materials when dyed at boil at atmospheric pressure.
ð They
alter the dispersing properties of the dyes and the physical characteristics of
the fibre so that more of the dyestuff can be transferred from the dye bath to
the fibre than in the absence of the carriers.
ð An
ideal carrier should be :-
(a) Availability
at low cost.
(b) Sufficiently
effective in increasing rate of dyeing.
(c) Absence
of unpleasant odour.
(d) Non
– toxic.
(e) Easily
removable after dyeing.
(f) Compatible
with dyestuffs.
(g) Low
volatility
(h) Unlikely
to cause any undue shrinkage of the material.
(i) Little
or no effect on the fastness of the final dyeing.
(j) Preferably
biodegradable.
ð Carriers
are mainly based on phenyl phenol (ortho and para) and di – or
trichlorobenzenes.
ð Orthophenyl
phenol may be used in the concentration range of 3 to 6 gpl. The free phenol is
insoluble in water and difficult to disperse in water.
ð To
overcome this defect, its sodium salt is used, but has no carrier action. The
free phenol should be liberated in the dye bath by the addition of acid –
liberating agent.
ð This
carrier adversely affects the light fastness of the final dyeing if not
completely removed from the dyeing.
ð Di
– and trichlorobenzenes are good carriers which promote dye absorption by the
fibre and the residual carriers do not affect the fastness of the final dyeing.
ð These
water insoluble carriers have to emulsify in the dye bath using an emulsifying
agent such as anionic detergent (alkyl benzene sulphonate).
ð These
carriers are toxic in nature and their use involves use of enclosed dyeing
machine.
ð Monomethyl
naphthalene has less powerful carrier action and is suitable only for dyeing
rapidly diffusing dyes. It is readily biodegradable.
ð Dimethyl
ester or Terephthalic acid possesses many desirable properties of an ideal
carrier but it has only moderate odour and it is costly.
ð Methyl
cresotinate is an efficient carrier and has no adverse effect on the light
fastness of the dyes. It is however expensive and volatile.
ð Butyl
benzoate is only moderate efficient in increasing the uptake of disperse dyes.
It is employed for promoting levelling in the high temperature dyeing of
polyester. It has a powerful odour.
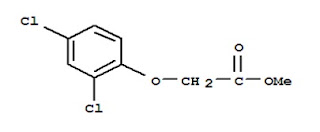
ð Methyldichlorophenoxy
acetate is a very efficient carrier with little odour, can be easily removed
from the dyed material. It is however very expensive.
Me = Methyl
Chemical
Formula of Methyldichlorophenoxy acetate
ð N
– Alkylphthalimide derivative has good efficiency, little odour and low
toxicity. Due to its ease of removal, it is especially useful in garment
dyeing.
Migration Inhibitor
ð Today,
continuous dyeing of textile fabrics are becoming more popular compare to batch
methods due to higher rate of production.
ð Continuous
dyeing methods require fabric to be dried after padding and before developing.
This step is known as intermediate drying.
ð Intermediate
drying is carried out on hot air machine, or drying cans. These machines for
drying operate by evaporating water from the fabric surface.
ð During
the passage of the fabric through the machine, the surface water is removed as
vapour and more water is drawn from the interior to the surface to restore
equilibrium between liquid and vapour.
ð Migration
of the dye particles to the surface may occur as a result of this movement of
water and lead to lack of penetration.
ð Also
differences in the rate of drying of the two faces of a fabric would give a two
– sided appearance because of unequal amount of dye, on the two surfaces.
ð This
difficulty of uneven dyeing due to migration can be avoided by the use of
migration inhibitors and also by careful drying.
ð In
the dyeing of vat dyes by the continuous pigment pad method migration
inhibitors such as common salt, glauber’s salt, sodium bisulphite, sodium
acetate and various thickeners such as gum tragacanth, sodium alginate are
added to the padding liquor.
ð Sodium
alginate, when neutral and very carefully prepared in concentration up to 1 g/l
is probably the most satisfactory. Sometimes carboxy methyl cellulose is
employed.
ð ICI
has recommended migration inhibitor V, which is said to be an organic anionic
polyelectrolyte. It produces level results and better colour yield.
ð Reactive
dyes are widely applied on cellulosic fabrics by continuous methods. In this
case migration inhibitors recommended are based on non – ionic
polyelectrolytes.
ð In
the thermosol process of applying disperse dyes to polyester/cellulosic
fabrics, it is absolutely necessary to have even distribution of the dyestuff
on the fabric prior to thermofixation, so migration inhibitors are added to the
padding liquor to prevent migration of dyestuffs during drying.
ð An
inhibitor to be suitable for thermosol process, should have the following
properties:-
(a) The
solid content of the additive should be as low as possible.
(b) It
should not affect the solubility or dispersion of the dyestuff.
(c) It
should prevent migration but should not hinder the diffusion of dye into fibre.
(d) It
should be resistant to high temperature.
(e) It
should be easily removable after dyeing.
Dye Fixing Agents
ð The
main drawback of direct cotton dyes is that their fastness to washing and
soaping is poor.
ð Attempts
have been made to develop some after-treatments which can improve the fastness
properties of these dyes on cellulosic fibres.
ð Cationic
surface active agents such as Fixanol C, Sandofix, Lyofix DF, Sapamines and
Hicofix GL are commonly used for this purpose.
ð This
after-treatment improves the fastness to water, acids and alkalis, but the
treated dyeing shows little or no improvement in solution of soaps or
detergent.
ð It
is known that these products function by forming a complex between the dye
anion and the cation of the agent.
ð The
complex being less soluble than the dye anion shows increased wet fastness.
ð This
complex, however, is broken down by anionic surface active agents leading to
poor fastness to soaping.
ð During
the dyeing of cotton with reactive dyes, two reactions proceed simultaneously
under alkaline conditions.
ð One
is the reaction of the dye with hydroxyl groups of cellulose, leading to the
fixation of dyestuffs, and the other is the reaction with hydroxyl groups of
water leading to the formation of the hydrolysed dye.
ð This
hydrolysed dye should be completely removed by soaping, as otherwise wash
fastness properties will be poor.
ð An
after-treatment with cationic agents such as Fixanol PN, has been suggested to
increase the fastness of the hydrolysed dye.
ð In
this case, the colour yield is also increased because the hydrolysed dye is
attached to the fibre by the cationic agent.
ð Recently,
an after-treatment with a base containing at least one primary or secondary
amino group e.g. diethanolamine has been also suggested for this purpose.
After Washing Agents
ð On
completion of dyeing, with most dyes it is usual to give an after-treatment
with a surfactant which is termed soaping, since soap was used for this
purpose.
ð Today
along with soap, a number of anionic and non – ionic surfactants are available
for this purpose.
ð In
the dyeing of cotton with vat dyes, soaping causes the aggregation of dye
particles, helps in developing true shades and removes surface dyestuff from
the fibre.
ð Soaping
is very important in azoic dyeing as it helps to improve the rubbing fastness.
ð In
case of the reactive dyes, soaping is important in order to remove unfixed
hydrolysed dye from the material and to obtain dyeing with satisfactory
fastness properties.
Stripping Agents
ð It
is sometimes necessary to remove the dye from dyed material in order to correct
the faulty dyeing. This process is called stripping.
ð The
primary requirements of a successful stripping methods are:-
(a) The
process must effectively remove the dyestuff
(b) The
fibre must remain substantially unimpaired so that it can be redyed to a
saleable item
(c) The
cost must be low.
ð Stripping
of direct dye on cotton is simple and consists essentially of treatment of the
material with either alkaline hydrosulphite or sodium chlorite.
ð Stripping
of vat dyes is difficult. An effective stripping agent for vat dyes must remove
the leuco compound as it is formed and leach it out into the bath, thus
preventing a re-adsorption on the fabric.
ð Cationactive,
long chain quaternary ammonium salts such as Lissolamine A and Lissolamine V
are found to be effective in stripping of vat dyes.
ð A
new agent based on polyvinyl pyrrolidone sold under the trade name Albigen A is
recommended for stripping vat, sulphur and direct dyes.
ð Stripping
of vat dyes is also achieved by using alkali and formamidine sulphinic acid,
prepared by the action of hydrogen peroxide on thiourea at near boiling point.
ð In
the stripping of azoic dye, certain quaternary ammonium compounds such as cetyl
trimethyl ammonium bromide in presence of alkaline reducing agent have been
found very effective.
ð Acid
dyes can be stripped from wool by treatment with acidified sodium sulphoxylate
formaldehyde, often in presence of a little formaldehyde.
ð Disperse
dyes from nylon are stripped by using either a mixture of non – ionic detergent
and polyvinyl pyrrolidone or sulphoxylate formaldehyde activated by acetic or
formic acid in presence of benzyl alcohol.
ð The
acid dye from nylon is stripped by using a mixture of trisodium phosphate and
non – ionic detergent, often in presence of benzyl alcohol.
ð Stripping
of disperse dyes from polyester is extremely difficult. Treatment with carrier
such as chlorinated benzene or salicylic acid is useful for partial stripping.
ð For
stripping deep shades on heat-set polyester, the following method is
recommended:- treat with carrier, zinc sulphoxylate and acetic acid at boil,
for 60 minutes, followed by a wash and another treatment with carrier sodium
chlorite and oxalic acid at boil for 45 minutes.